|
 |
Melanotaenia splendida [Daintree River] - photo© Gunther Schmida |
(Peters, 1866)
Eastern Rainbowfish
Species Summary
Melanotaenia splendida subsp. splendida were originally collected from the Fitzroy River in central Queensland and scientifically described as Nemacentrus splendida in 1866. Gerald Allen's revision of the family Melanotaeniidae in 1980 places them under their current name. The different subspecies of Melanotaenia splendida are not easily identified in relation to each other as they display a great variation of colours and markings. Principal visual differences are body depth and colour pattern, which is variable depending on location and natural environment. At the same time, body form within each subspecies is variably and appears to be related to habitat conditions, which can sometimes make correct identification difficult. Populations of almost every river system they occupy have their own distinctive body colour and pattern. Geographic distribution is very helpful; if you know where they were collected you can generally make a confident identification. Consequently, specific names usually based on the locality where each is found are used by rainbowfish enthusiasts to identify each variety. Where populations need to be identified, they are often done by inclusion of a form or population identifier in brackets following the species name e.g., Melanotaenia splendida subsp. splendida (Burdekin River).
M. s. splendida display a great natural assortment of colours. The basic body colouration is overall pale bluish-green, olivaceous to yellowish, grading to white on the lower sides. Each horizontal scale row is separated by a narrow orange to reddish stripe. The scales on the side of the body usually have a bluish-green, yellowish-red or purplish sheen. The mid-lateral stripe can be faded black to deep yellowish anteriorly, and bluish-green or brownish-green on the caudal peduncle. Other body stripes can be yellow, green, blue or red. There is usually an orange or yellow spot on the opercula. The dorsal, caudal and anal fins can be red and yellow chequered or orange-yellow with bright red spots on their membranes, with faint black edges. Other forms can have a blue-green body with yellow-green fins, with dark flecks and a dark border. However, colour is extremely variable and will depend upon the mood of the fish, water conditions and diet. Females and juveniles have plain silvery bodies and fins that are either translucent or only faintly coloured compared to the brighter colours of males.
Genetic studies beginning in the mid 1990's (Zhu et al. 1994) revealed the existence of significant genetic variation between populations of M. s. splendida that occur in the upland streams of north Queensland. In particular, these studies highlighted the degree of isolation of upland populations from the lowland populations. Subsequent genetic research (McGuigan et al. 2000) suggested that at least some of these species are unusual variants of Melanotaenia splendida - or populations displaying genes that have traits of more than one species. As a direct result of some of this research, the Utchee Creek Rainbowfish (Melanotaenia utcheensis) was described as a new species in 2000, with populations known from Utchee, Fisher, Rankin and Short Creeks in the North and South Johnstone River catchments (McGuigan 2001).
Rainbowfish from upstream sections of the Burdekin River have long been considered to be a distinct species by rainbowfish enthusiasts, and are known in the hobby as the Burdekin Rainbowfish (Running River or "zigzag" form). This form is believed to also be present in other tributaries draining the Paluma Range, notably the Fanning River. There are other informally recognised forms of "splendida" such as the Davies Creek Rainbowfish, Kuranda Reds and Mena Creek Rainbowfish. Despite the research that has been undertaken to date, the specific status and distribution of M. s. splendida still remains unclear.
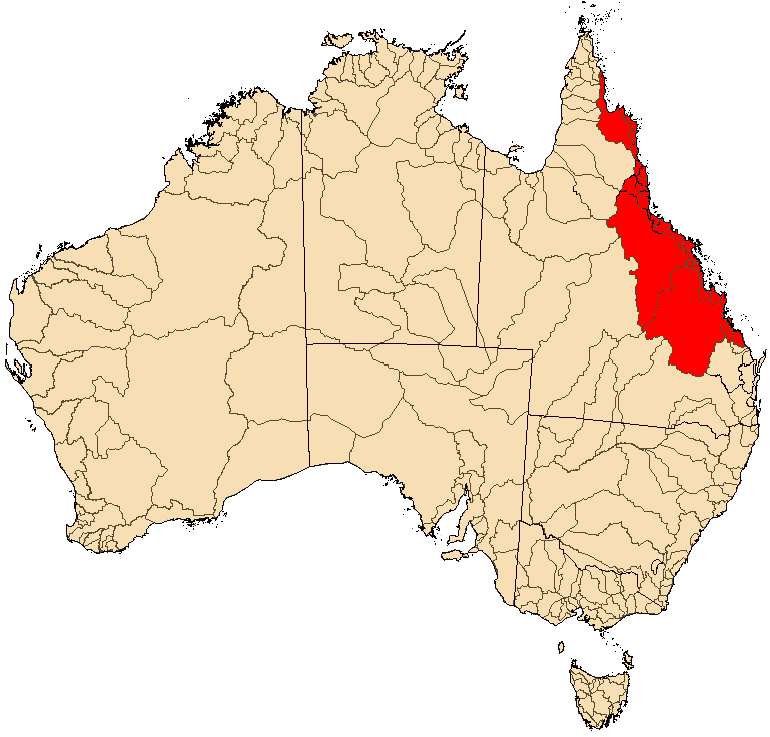 |
Distribution Map |
Distribution & Habitat
M. s. splendida are found in streams east of the Great Dividing Range along the coast of Queensland from Deepwater Creek a small coastal stream located between the cities of Bundaberg and Gladstone extending up the east coast to around the Lockhart and Stewart Rivers on Cape York Peninsula. Scrubby Creek, just south of the Lockhart River, appears to be the northernmost location for this species.
M. s. splendida are usually abundant in almost every kind of freshwater habitat, from slow-moving streams, swamps, lakes and clear flowing rivers. They are most abundant in open reaches of zero to low flow containing abundant instream vegetation and cover, and an intact riparian zone. They are less abundant in riffle/rapid habitats. Abundance varies significantly over the seasons; being greatest after the wet season (which enhances recruitment) but will decline greatly as flow decreases during drought condition. They are frequently found in company with M. maccullochi, M. trifasciata, Cairnsichthys rhombosomoides, and Pseudomugil species. Their natural environment is subjected to seasonal variations with water temperature (12-36°C), pH (5.0-9.2), and hardness levels varying considerably. This wide range of water conditions matches the wide distribution of the species.
Melanotaenia duboulayi inhabits the coastal drainages of the east coast of northern New South Wales from the Hastings River to Baffle Creek, north of the Bundaberg region in Queensland. It may be that these two species live sympatrically in some locations. Natural hybrids of M. splendida and M. duboulayi have been reported from Deepwater Creek and Mullett Creek.
 GS1.jpg) |
Melanotaenia splendida [Wallaby Creek, Annan River] - photo© Gunther Schmida |
Biology & Ecology
Not a lot is known about the biology of M. s. splendida in their natural habitat. Most information is mainly based on aquarium observations. In captivity they can reach a maximum size of 12 cm, but are usually less than 8 cm. Males are more brightly coloured, larger, and much deeper bodied than females. Generally, the larger males can usually be identified from the elongation of posterior rays in the second dorsal and anal fins. Sexual maturity occurs at about 3~4 cm for both sexes.
In their natural environment M. s. splendida has a prolonged spawning period with a peak of spawning activity in pre-flood and flood periods, although individuals in spawning condition and juveniles may be found throughout the year. Spawning during the wet season (November to April), when the inundation of streams and floodplains ensures an expanded habitat (in area and diversity) and a greater array and abundance of food. In contrast, spawning peaks during the dry season (May to October) ensures that larvae are produced during a period of relatively stable environmental conditions. This strategy increases the chances of some eggs surviving. Increased stream flow may result in conditions unfavourable for reproduction (i.e., physical removal of eggs, larvae and spawning substrate). In the main, M. s. splendida will breed when environmental conditions ensure maximum fertilisation and larval survival. They usually spawn small numbers of eggs over a large area in slow-flowing waters and the backwaters of flooded areas. The presence of extensive spawning substrate enables them to 'spread the risk' from predators. The eggs are attached by adhesive threads to aquatic plants and other objects in the water, which hide them from predators. The eggs, however, are subject to desiccation if the water level drops or to dispersal if there is a flood.
In captivity, with limited area and artificial substrate, females may spawn all their eggs at the same time. Spawning occurs predominantly in the early morning, with one to three eggs deposited at a time, during which time 15~100 eggs can be produced. The number of eggs shed by a single female is directly related to the size of the female with large females spawning from 40~250 eggs. Females usually only spawn once each day; however, males will often spawn with more than one female in one day.
 GS.jpg) |
Melanotaenia splendida [Clohesy River, Kuranda] - photo© Gunther Schmida |
The eggs of M. s. splendida at fertilisation are similar in appearance to those of other rainbowfishes. All are spherical, with a number of adhesive filaments, 3–8 mm in length, arising from a small area of the chorion at the animal pole. Spawned eggs, which range in size from 0.93–1.24 mm in diameter, are adhesive, negatively buoyant in freshwater and are usually clear to light amber in colour. The eggs hatch after an incubation period of four to nine days depending on temperature. Temperature is one of the major factors that influences the embryonic period for rainbowfishes. Average embryonic period is about 5 days at 28°C.
The average larval length of M. s. splendida at hatching ranges from 2–4 mm, which is similar to other rainbowfish species. Hatched larvae are well developed and competent swimmers. Growth rates of the larvae are initially slow, with little variation until around 7–14 days. Growth is directly related to the initial absorption of the yolk sac and the provided larval diet. After that period growth rates increased. As the larvae increased in age, the variation in length between individuals also increased.
The continued growth and development of the fry will vary from one hobbyist to another and is largely conditional upon captive conditions such as temperature, water quality, and feeding regime. Under aquarium conditions increased temperature generally results in higher growth rates. 28 ± 1°C is considered the most effective and safe temperature for optimum growth rate. At this temperature range, M. s. splendida are relatively fast growing with sexual differences beginning to appear between 9 and 12 weeks after hatching.
Food is an important factor affecting growth, especially in the early larval stages. Research has found that diet strongly affects not only fecundity but also the biochemical make-up of eggs and sperm as well as the growth rate and survival of larvae. The preferred size of food for larval fishes increases as mouth size and feeding competency increase. Providing natural 'green-water' (phytoplankton) with resident zooplankton as food for the newly hatched fish has several advantages. The larvae are easily able to switch to different sized food, a feature not present when feeding foods such as rotifers or brineshrimp. Green water also enables the zooplankton to feed on resident algae and microbes, thus retaining their nutritional value for greater periods of time. In addition, a varied diet may affect the growth of rainbowfishes positively.
GES.jpg) |
Melanotaenia splendida [Bowen] - photo© Gunther Schmida |
Remarks
Because of the great variation in colours and body forms, M. s. splendida should be bred within their own localised groups. Regardless of their various colour patterns, at this point of time, they are all believed to belong to the same species and are capable and willing to breed together if permitted to do so. The serious hobbyist intent on maintaining pure lines must keep every variety in separate aquariums. Unless this is done, members of the different varieties will interbreed and complicate future breeding programs and identification.
Literature
Allen G.R., S.H. Midgley and M. Allen. (2002). Field Guide to the Freshwater Fishes of Australia. Western Australian Museum / CSIRO Publishing, Perth, 394 pp.
Badger A.C. (2004). The effects of nutrition on reproduction in the Eastern Rainbowfish, Melanotaenia splendida splendida. Masters (Research) thesis, James Cook University.
Beumer J.P. (1979). Reproductive cycles of two Australian freshwater fishes, the spangled perch, Therapon unicolor Gunther, 1895 and the east Queensland rainbowfish Nematocentris splendida Peters 1866. Journal of Fish Biology 15: 111-34.
Humphrey C., D.W. Klumpp and R.G. Pearson (2003). Early development and growth of the eastern rainbowfish, Melanotaenia splendida splendida (Peters) I. Morphogenesis and ontogeny. Marine and Freshwater Research 54: 17-25
Kefford B.J., J.E. Dunlop, N. Horrigan, L. Zalizniak, K.L Hassell, R. Prasad, S. Choy and D. Nugegoda (2006). Predicting salinity-induced loss of biodiversity. Project No: RMI 12 Final Report to Land and Water Australia.
Leggett R. (1996). Salted Rainbows, the perfect hors d'oeuvre. Fishes of Sahul, 10 (1): 443-444.
McGuigan K., D. Zhu, G.R. Allen and C. Moritz (2000). Phylogenetic relationships and historical biogeography of melanotaeniid fishes in Australia and New Guinea. Marine and Freshwater Research 51: 713-723.
McGuigan K.L. (2001). An addition to the rainbowfish (Melanotaeniidae) fauna of north Queensland. Memoirs of the Queensland Museum 46: 647-655.
Peters W. (C. H.) 1866. Mittheilung über Fische (Protopterus, Auliscops, Labrax, Labracoglossa, Nematocentris, Serranus, Scorpis, Opisthognathus, Scombresox, Acharnes, Anguilla, Gymnomuraena, Chilorhinus, Ophichthys, Helmichthys). Monatsberichte der Akademie der Wissenschaft zu Berlin 1866: 509-526.
Zhu D., B.G.M. Jamieson, A. Hugall and C. Moritz (1994). Sequence evolution and phylogenetic signal in control-region and cytochrome b sequences of rainbow fishes (Melanotaeniidae). Molecular Biology and Evolution 11: 672-683.
Adrian R. Tappin
Updated April, 2013



|
|

